Abstract
INTRODUCTION
Central nervous system (CNS) relapse is a challenging situation in diffuse large B-cell lymphoma (DLBCL). High CNS-International Prognostic Index (IPI), activated B-cell (ABC) subtype and MYD88 L265P mutation, features often found in transformed Waldenström macroglobulinemia (WM), are associated with a higher risk for developing CNS relapse. This study was aimed to describe CNS involvement in a large cohort of transformed WM.
METHODS
This international multicenter retrospective study included patients with a diagnosis of WM and a concurrent or sequential histological diagnosis of DLBCL. CNS disease was diagnosed by detection of DLBCL cells in the cerebrospinal fluid and/or by brain biopsy. Patients with CNS involvement by lymphoplasmacytic cells (Bing-Neel syndrome) were excluded. Of 254 identified patients with a diagnosis of histological transformation (HT) between 1988 and 2020, 19 were excluded due to lack of data on extranodal involvement. The first part of the analysis focused on baseline CNS involvement. Clinicobiological characteristics were compared between groups using Chi-square or Fisher's exact tests or Mann Whitney tests as appropriate. We analyzed CNS recurrence in the second part of the study. Forty-eight additional patients were excluded due to baseline CNS involvement (n = 25), absence of treatment at HT (n = 14) and lack of details on follow-up (n = 9). Cumulative incidence of CNS relapse was analyzed using competing-risk models that accounted for other events like systemic relapse or death from any cause, reporting sub-hazard ratio (SHR).
RESULTS
Baseline CNS involvement was present in 25 patients (11%) with transformed WM, including 10 (4%) with parenchymal disease, 10 with leptomeningeal, 4 (2%) with both, and 1 with unspecified CNS involvement. Characteristics associated with baseline CNS involvement were performance status 2-4 (P=0.03) and ≥2 extranodal sites (P=0.02). Median survival after HT was 1 year [0.7-2.5], comparable to the one of patients without CNS disease (1.8 year [1.2-2.6], P=0.74). We observed no difference in survival based on isolated CNS involvement (n = 10) compared to CNS and systemic involvement (n = 15) (P=0.94).
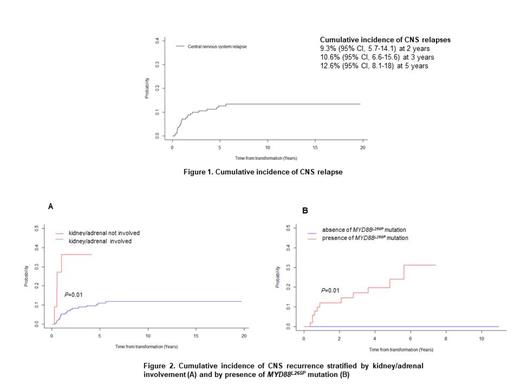
Twenty-three CNS relapses occurred (12%). The 2-year and 3-year rates of CNS relapse were 9% (95% CI, 6-14) and 11% (95% CI, 7-16) (Figure 1). The median time to relapse in the CNS was 11 months (95% CI, 7-25). Thirteen CNS recurrences (57%) occurred during the first year of follow-up. Seventy percent were isolated CNS relapses. The location was leptomeningeal in 43% of cases, parenchymal in 35%, both in 17%, and unspecified in 4%. According to CNS-IPI risk groups (data available for 20 patients), 9 patients (45%) belonged to the high-risk group, 10 (50%) to the intermediate-risk group and 1 (5%) to the low-risk group. Prior to CNS relapse, 87% of patients had received rituximab, and 39% had received CNS prophylaxis (30% intrathecal chemotherapy, 4% high-dose methotrexate (HD-MTX), and 4% both). After CNS recurrence, 96% of patients received salvage treatment: combination of HD-MTX and HD-cytarabine (48%), HD-MTX alone (30%), or HD-cytarabine alone (9%). Four patients underwent consolidative autologous stem cell transplantation. The median survival after CNS relapse was 5.6 months. Factors associated with 3-year cumulative incidence of CNS recurrence in univariate analysis were involvement of kidney/adrenal glands (HR, 4.4; P=0.01) and MYD88 L265P mutation (P=0.01) (Figure 2A and B). Of note, among 74 patients (over 187, 40%) with data available for MYD88 mutation status, 11 CNS relapses occurred in patients with MYD88 L265P mutation (n = 54, 20%) whereas no relapse were observed in MYD88 WT cohort (n = 20). A trend toward higher risk of CNS relapse for ≥2 extranodal sites (HR, 2.3, 95% CI 0.98-5.3; P=0.06) was observed. Cumulative incidence according to CNS-IPI risk groups (0% in the low-risk, 9% in the intermediate-risk and 14% in the high-risk group) was not statistically significant (P=0.47).
CONCLUSION
CNS involvement occurs frequently in transformed WM. Rate of CNS relapse seems similar to DLBCL patients belonged to the CNS-IPI high-risk group. Special attention should be paid to patients with kidney/adrenal involvement and MYD88 L265P mutation.
Vos: Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: Travel reimbursement. Treon: X4: Research Funding; Janssen: Consultancy, Research Funding; Dana Farber Cancer Institute: Current Employment; BMS: Consultancy, Research Funding; Self: Patents & Royalties: Holder of multiple patents related to testing and treatment of MYD88 and CXCR4 mutated B-cell malignancies; AbbVie: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Dimopoulos: Janssen: Honoraria; Beigene: Honoraria; Amgen: Honoraria; BMS: Honoraria; Takeda: Honoraria. Kapoor: Cellectar: Consultancy; Karyopharm: Consultancy; BeiGene: Consultancy; Pharmacyclics: Consultancy; Sanofi: Consultancy; Amgen: Research Funding; Ichnos Sciences: Research Funding; Regeneron Pharmaceuticals: Research Funding; Glaxo SmithKline: Research Funding; Karyopharm: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; AbbVie: Research Funding. Castillo: Abbvie: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; Roche: Consultancy; TG Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal